 |
 |
Dr. Robi Hossan - Microsystems Engineering Lab |
| Research Areas |
| Our research focuses on the theoretical investigation and development
of integrated microdevices for i) biomarker protein detection, ii) bioparticle assembly and interactions and
iii) cell separation and trapping for biomedical applications. We also
investigate fundamental aspect of fluid flow and pressure losses in
microchannel with second law perspective specially the correlation between
entropy generation and mixing efficiency in microfluidics and electromagnetic heating. Since our research are multidisciplinary in nature, we invite and have been attracting students and researchers with diverse background for extensive collaborations. Our lab has micro/nano fabrication facilities for microdevice development. Please contact Dr. Hossan for details of micro fabrication capabilities and potential collaborations. |
| Biomarker Protein Detection in Microfluidic Devices |
| One
of the major challenges in bio-sensing is the low-abundance of
biomarker molecules/proteins in highly diluted analytes, which is often
the
most crucial in a biological standpoint; especially for early diagnosis
of
diseases. For example, the biomarker proteins – the cTnI isoforms –
that are
related to cardiac arrest or heart failure are present so vanishingly
small
concentration in a relatively small amount of blood that it is very
difficult
to detect. So concentration of bio-molecules prior to analysis is
critical in
development of integrated multifunctional lab-on-a chip systems or
micro-nanofluidic
devices. The goal of our research is to integrate preconcentration and
separation steps (isotachophoresis - a non linear electrophoresis
technique) with current immunoassay protocols (immobolization and
antibody signal amplification) in a microfluidic platform. Specifically
in this project we are working with cardiac protein troponin I isoforms
which is considered as one of the most reliable biomarker for acute
myocardial infarction (MI). |
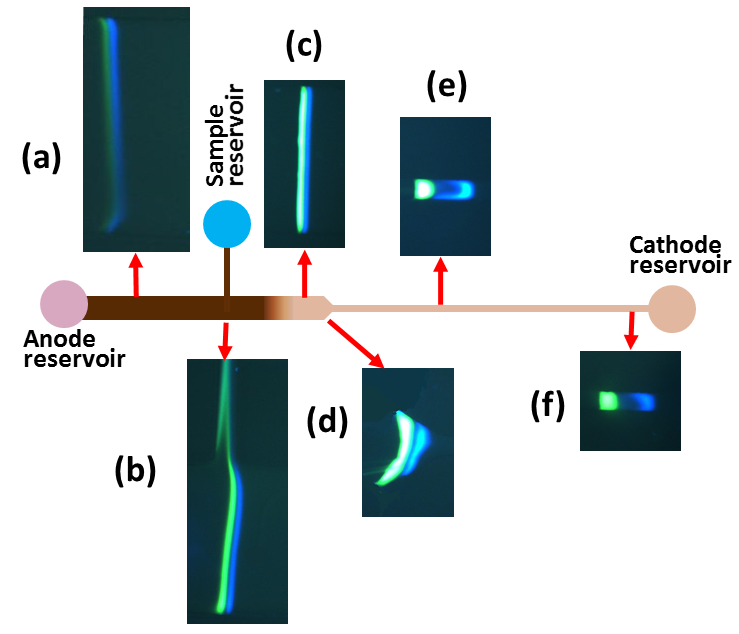 |
Figure:
Time
series photos of stacked proteins during isotachophoresis in a gradual 2D step
reducing microchannel. The width and depth of the channel varies with location.
(a) the proteins starts to stacking (b) the proteins have gathered most of its
total mass and some sample is lost due to migration through T-junction (c) the
proteins have experienced depth change with 10x reduction and protein bands
become more vivid d) the proteins are passing through the width change and two
distinct bands become wider d) the proteins are in the narrower channel with
100x reduction and bands become more distinct with some separation (f) the
proteins have stacked and concentrated with little separation.
|
| Further Reading: 1. Jubery, T.Z., Hossan, M.R., Bottenus, D.R., Ivory, C.F., Dong, W., Dutta, P., "A new Fabrication Technique to form Complex Polymethylmethacrylate microchannel for bioseparation", Biomicrofluidics, 6, 016503, 2012 2.Bottenus, D.R., Hossan, M.R., Quyang, Y., Ivory, C.F., Dong, W., Dutta, P. "Preconcentration and detection of the phosphorylated forms of cardiac troponin I in a cascade microchip by cationic isotachophoresis", Lab on a Chip, 11, 3793-3801, 2011 |
| DEP Particle-Particle Interactions and Assembly |
|
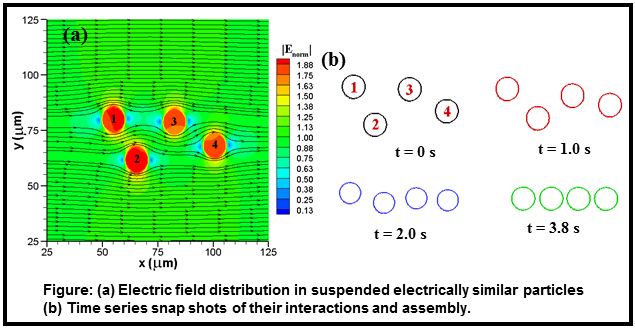
In dielectrophoresis (DEP), an applied electric field polarizes
dielectric particles or materials and hence causes a net force due to unequal
electric fields on the accumulated charges. DEP works both on charged and
neutral particles. We developed a hybrid immersed boundary-immersed interface
numerical method to study DEP particle-particle interactions, assembly and
transport in microfluidic devices. Our research reveals that within a close
proximity, particles form a chain (either parallel to electric field or perpendicular
to the electric field) regardless of initial orientation, locations, size and
electrical properties. Our study also proposed a microfluidic design where
particles can be assembled in a junction of microfluidic device. |
 |
| Further Reading: 1. Hossan, M. R.,Dillon, R., Roy, A.K., Dutta, P., "Modeling and Simulation of Dielectrophoretic Particle-Particle Interactions and Assembly", Journal of Colloid and Interface Sciences, 394, 619-629, 2013 2. Hossan, M.R., Dillon, R., Dutta,"Hybrid Immersed Interface-Immersed Boundary Methods for AC Dielectrophoresis", Journal of Computational Physics, 270, 640-659, 2014 3. Hossan, M. R.,Gopmandal, P.P., Dillon, R., Dutta, P., "Bipolar Janus Particle Assembly in Microdevices", Electrophoresis, 2015, DOI: 10.1002/elps.201400423 |